1 mol nacl to grams|how many grams are in 1.946 moles of nacl : Cebu Do a quick conversion: 1 grams NaCl = 0.017110756386119 mole using the molecular weight calculator and the molar mass of NaCl. All Chapters. Is This Hero For Real?, Chapter 102. Is This Hero For Real?, Chapter 101 – END. Is This Hero For Real?, Chapter 100. Is This Hero For Real?, Chapter 99.
0 · number of moles in nacl
1 · how many moles are in 80.00 grams of table salt nacl
2 · how many grams are in 5.00 mol of sodium chloride
3 · how many grams are in 1.946 moles of nacl
4 · how do you convert grams to moles
5 · grams to molecs
6 · convert mole of nacl to grams
7 · More
8 · 1.50 g nacl is equivalent to 0.0257 mol what the molari
Robô pega letras. O Robô Pega Letras precisa de ajuda para coletar as letrinhas das palavras que aparecem na tela. Mas atenção: você precisa coletar as letras certas, senão volta para o começo do jogo. Vamos treinar seu conhecimento das letras e esquivar dos perigos, como as empilhadeiras e prensas.
1 mol nacl to grams*******1 moles NaCl to grams = 58.44277 grams 2 moles NaCl to grams = 116.88554 grams 3 moles NaCl to grams = 175.32831 grams 4 moles NaCl to grams = 233.77108 grams 5 moles NaCl to grams = 292.21385 grams 6 moles NaCl to grams = 350.65662 grams 7 moles NaCl to grams = 409.09939 grams 8 . See more
How many moles NaCl in 1 grams?The answer is 0.017110756386119.We assume you are converting between moles NaCl and gram.You can view more details . See more
You can do the reverse unit conversion fromgrams NaCl to moles, or enter other units to convert below: See moreIn chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the . See more
Do a quick conversion: 1 grams NaCl = 0.017110756386119 mole using the molecular weight calculator and the molar mass of NaCl.
This free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams.Do a quick conversion: 1 moles Sodium Chloride = 58.44277 gram using the molecular weight calculator and the molar mass of NaCl.how many grams are in 1.946 moles of nacl 751K subscribers. Subscribed. 256. 62K views 5 years ago. In this video well learn to convert moles of NaCl to grams. The technique used can be applied to any mole to gram conversion. For our.
Convert grams to moles and moles to grams using a simply calculator. Plus, learn the g to mol formula and how to account for molar mass.This grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. The molar mass of NaCl is 58.44 g/mol. To get this, sum up the molar masses of 1 sodium atom and 1 chlorine atom: 1 × 22.99 g/mol + 1 × 35.45 g/mol = . You can calculate the molar mass of a molecule by summing the molar masses of the composing atoms with the help of the periodic table. Use this grams to . This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). You can also .Molar mass can then be used as a conversion factor to convert amounts in moles to amounts in grams. It is important to remember that “mol” in this expression refers to moles of solute and that “L” refers to liters of .In the previous section, several relationships were written, including:. 1 mol Al = 26.98 g Al = 6.022 × 10 23 atoms Al ; 1 mol C 12 H 22 O 11 = 342.3 g C 12 H 22 O 11 = 6.022 × 10 23 molecules C 12 H 22 O 11; 1 mol NaCl = 58.44 g NaCl = 6.022 × 10 23 formula units NaCl; These relationships may be used to convert from grams to moles or vice versa; or from .
Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol. If you know how to calculate molar mass, learn about other ways to express the .Now we have to perform moles to grams calculation: Molar Mass = 107.868. (for calculations, tap Molar Mass Calculator) By using moles to grams formula: m = n ∗ M. m = 38.99 ∗ 107.868. m = 4205.77332 g. You can also verify the results by fetching the values in our free moles to grams calculator.
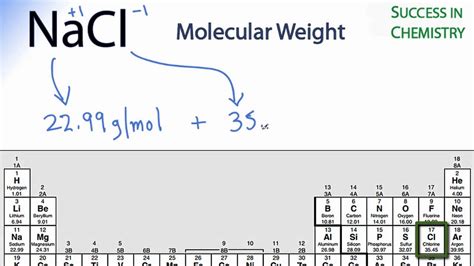
For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams. How to calculate the molecular weight of NaCl? Molar mass of NaCl = 58.44277 g/mol. This compound is also known as Sodium Chloride. Convert grams NaCl to moles or moles NaCl to grams. Molecular .David Park. 4 years ago. First, you can calculate the molar mass of FeCl2 by adding the molar masses of Fe (55.845 g/mol) and 2 atoms of Cl (2 times (35.446 g/mol). This gives a molar mass of 126.737 g/mol. Since each mole is 126.737 grams, you multiply 3.5 mols by 126.737 grams, giving you 443.58 grams.The molecular weight of NaCl is 58.44 grams/mole. If you had a 1.0 molar solution (1.0 M), you would have to put 58.44 g of salt in 1.0 liter of solution. How many moles of NaCl would you have in 100 mL of this solution?
There are 4 easy steps to find the molar mass of NaCl based on its chemical formula. 1. Count The Number of Each Atom . Convert NaCl From Grams to Moles and Molecules. Weight g. Convert to Moles. Composition of Sodium Chloride - NaCl . Element Symbol Atomic Mass # of Atoms Mass Percent; Sodium: Na: 22.9898 g/mol: 1:How many grams NaCl in 1 mol? The answer is 58.44277. We assume you are converting between grams NaCl and mole. You can view more details on each measurement unit: molecular weight of NaCl or mol This compound is also known as Sodium Chloride. The SI base unit for amount of substance is the mole. 1 grams NaCl is equal to .1 mol nacl to gramsMore information from the unit converter. How many moles (NaCl) in 1 grams? The answer is 0.017110756386119. We assume you are converting between moles (NaCl) and gram.You can view more details on each measurement unit: molecular weight of (NaCl) or grams The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles .More information from the unit converter. How many moles NaCl in 1 grams? The answer is 0.017110756386119. We assume you are converting between moles NaCl and gram.You can view more details on each measurement unit: molecular weight of NaCl or grams The molecular formula for NaCl is NaCl.The SI base unit for amount of substance is the .Question 3 The MW of NaCl = 58 grams/mol. The MW of KCl is 74 grams/mol. What weighs more, 1 mol of NaCl or 1 mol of KCL? КСІ Naci Thevad Question 5 The MW of NaCl = 58 grams/mol. The MW of KCl is 74 grams/mol. What has more molecules, 100 grams of NaCl or 100 grams of KCI? 100 grams of NaCl 100 camer Neither, they are . The mass of 1.84 mol NaCl is found by multiplying the number of moles (1.84 mol) by the molar mass of NaCl (58.44 g/mol) to get 107.53 grams, rounded to three significant figures. Explanation: To calculate the mass of 1.84 mol NaCl, we use the molar mass of NaCl which is given as 58.44 g/mol.1 mol nacl to grams how many grams are in 1.946 moles of nacl Assume we want to dissolve 70.128 grams of salt in 1.5 kg of water. So: moles of NaCl = 70.128 g / (58.44 g/mol) = 1.2 mol. Plug the number of moles and the mass of the solvent into the molality formula. Divide 1.2 mol by 1.5 kg, and you'll find out that the molality of the NaCl solution is 0.8 molal (in standard molality units: 0.8 mol/kg).More information from the unit converter. How many moles NaCl in 1 grams? The answer is 0.017110756386119. We assume you are converting between moles NaCl and gram.You can view more details on each measurement unit: molecular weight of NaCl or grams The molecular formula for NaCl is NaCl.The SI base unit for amount of substance is the .
Question 3 The MW of NaCl = 58 grams/mol. The MW of KCl is 74 grams/mol. What weighs more, 1 mol of NaCl or 1 mol of KCL? КСІ Naci Thevad Question 5 The MW of NaCl = 58 grams/mol. The MW of KCl is 74 grams/mol. What has more molecules, 100 grams of NaCl or 100 grams of KCI? 100 grams of NaCl 100 camer Neither, they are .
The mass of 1.84 mol NaCl is found by multiplying the number of moles (1.84 mol) by the molar mass of NaCl (58.44 g/mol) to get 107.53 grams, rounded to three significant figures. Explanation: To calculate the mass of 1.84 mol NaCl, we use the molar mass of NaCl which is given as 58.44 g/mol. Assume we want to dissolve 70.128 grams of salt in 1.5 kg of water. So: moles of NaCl = 70.128 g / (58.44 g/mol) = 1.2 mol. Plug the number of moles and the mass of the solvent into the molality formula. Divide 1.2 mol by 1.5 kg, and you'll find out that the molality of the NaCl solution is 0.8 molal (in standard molality units: 0.8 mol/kg). First, convert grams of NaCl to moles of NaCl. From the periodic table: Na = 23.0 g/mol; Cl = 35.5 g/mol; . (1 mole / 74.6 g) * 3 grams = 3 / 74.6 = 0.040 moles Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water (assuming a density of 1 g/ml), but you also have 3 grams of solute, so .How many grams NaCl in 1 mol? The answer is 58.44277. We assume you are converting between grams NaCl and mole. You can view more details on each measurement unit: molecular weight of NaCl or mol The molecular formula for NaCl is NaCl. The SI base unit for amount of substance is the mole. 1 grams NaCl is equal to 0.017110756386119 .How many grams of NaCl are contained in 0.250 L of a 5.30-M solution? Solution The volume and molarity of the solution are specified, . In Example 3.17, the molar amount of NaCl computed in the first step, 1.325 mol, would be properly rounded to 1.32 mol if it were to be reported; however, although the last digit (5) is not significant, it . 1 mole of NaCl = 58.44 grams ( the molar mass of Na which is 22.99 g/mol + the molar mass of chlorine which is 35.45 g/mol = 58.44 g/mol). This amount is the placed in a 1 liter volumetric flask is precisely calibrated to hold a 1 liter solution at room temperature. Graduated cylinders do not have precise volumes as volumetric flasks . How many grams of NaCl are in 1.92 moles of NaCl? Every mol of NaCl contains a mol of Na, weighting 23 grams, and a mol of Cl, weighing 35.5 grams. So, every mol of NaCl weights 58,5 (=23+35.5) grams.Calculate the molar mass of (NaCl) in grams per mole or search for a chemical formula or substance. Molecular weight of (NaCl) (NaCl) molecular weight. Molar mass of (NaCl) = 58.44277 g/mol. Convert grams (NaCl) to moles. or. moles (NaCl) to grams. Molecular weight calculation: (22.98977 + 35.453)

The molar mass of CoCl 2 •2H 2 O is 165.87 g/mol. Therefore, molesCoCl2 ⋅ 2H2O = ( 10.0g 165.87g / mol) = 0.0603mol. The volume of the solution in liters is. volume = 500 mL( 1L 1000mL) = 0.500L. Molarity is the number of moles of solute per liter of solution, so the molarity of the solution is.For example, let’s calculate the mass of 2 moles of table salt, sodium chloride or NaCl. The molar mass of sodium chloride is 58.44 g/mol, which is the sum of the atomic masses of 1 mole of sodium (Na, 22.99 g/mol) and 1 mole of chlorine (Cl, 35.45 g/mol). Two moles of table salt, NaCl, would be twice this amount: m = 2 × 58.44 m = 116.88 g
WEB19 de jun. de 2022 · Esperamos por vocês! 💖🥰 Investigamos o Enaldinho e a Aninha e vimos o que eles fazem quando estão sozinhos! Pegamos eles no flagra e ficamos .
1 mol nacl to grams|how many grams are in 1.946 moles of nacl